Polysaccharides are carbohydrates formed by more than 9 monosaccharides linked by glycosidic bonds.
When they are formed by the same kind of monosaccharides, they are called homopolysaccharides, like starch, glycogen and cellulose, formed each of them by hundreds of molecules of glucose linked by glycosidic linkages.
If the polysaccharides molecules are formed by different kinds of monosaccharides, they are considered heteropolysaccharides. Hyaluronic acid, formed by thousands of alternative units of N-acetyl glucosamine and glucuronic acid, is an example of heteropolysaccharide.

HOMOPOLYSACCHARIDES:
Cellulose
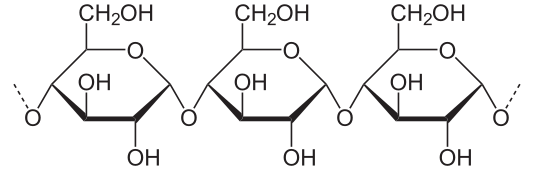
Cellulose is a linear polymer of D-glucose residues bonded by b(1, 4)-O-glycosidic linkages. It is the most abundant carbohydrate in nature.
It is formed by glucose units, linked by Beta-1, 4 O-glycosidic linkages. We can say then that, if we consider the kind of linkage, the repeating unit in cellulose is cellobiose, the disaccharide formed by two molecules of glucose linked by Beta-D-O glycosidic bonds, (that is why some text books say that the monomer in cellulose is cellobiose).
The long fibers of cellulose are held together by intermolecular hydrogen bonds. Hydrogen bonding continues in the same plane with other chains as well as in planes above and below this plane to form strong, fibrous bundles. It made cellulose very appropriate for its structural function in plants
Since cellulose is formed by glucose molecules, it can be a source of energy for certain species. The lack in human beings of appropriate enzymes for digesting cellulose make this polysaccharide unsuitable for human nutrition (Have you though about how hunger in the world could disappear if we had enzymes for digesting cellulose?). Cellulose and derivatives are used as a component of laxatives for humans.
Starch:
Starch is the second most abundant carbohydrate in nature.
The biological functions include, in plants, the main way of storage of sugar, and consequently, of energetic sources; in humans, the first supply of glucose on diet (Answer to C-O7)
Starch is not really a molecule, but a grain formed by two different kinds of molecules: Amylose and Amylopectin
Amylose
Amylose is a linear molecule formed by glucose units linked by alpha-1, 4 O glycosidic linkages. Taking in account the kind of linkage we can say that the repeating unit in amylose is maltose. (It explains that some books indicate that the monomeric unit in amylose is maltose).
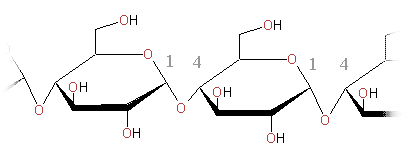
Amylose molecule is helicoidal
Amylopectin
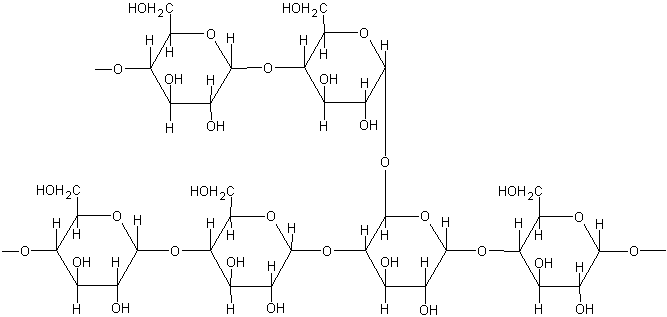
Amylopectin is the second type of molecule that forms starch. It is a branched molecule, formed also by glucose. Amylopectin contains D-glucose residues bonded together by a(1, 4)-O-glycosidic linkages with branching through a(1 6)-O-glycosidic linkages.

The disaccharides that can be obtained from the digestion of amylopectin are maltose and isomaltose.
Amylopectin shows a branch each 24-30 units of glucose,

Glycogen
The structure of glycogen is very similar to amylopectin but more branched, with one branch every 8 to 12 glucose unit
Glycogen is the way in which glucose is stored in animals. Glycogen is stored mainly in liver (to release glucose to blood when necessary) and in muscle, where it is used as a reserve of energy for muscular contraction (Answer to C-o8)
HETEROPOLYSACCHARIDES
Heteropolysaccarides contain two or more different kind of monosaccharides. Usually they provide extracellular support for organisms of all kingdoms: the bacteria cell envelope, or the matrix that holds individual cells together in animal tissues, and provides protection, shape and support to cells, tissues and organs.
Heteropolysaccharides provide extracellular support to very different organisms, from bacteria to humans; together with fibrous proteins, like collagen, elastin, fibronectin, laminin and others, heteropolysaccharides are the most important components of the extracellular matrix. Hyaluronic acid, condroitin sulfates and dermatan sulfates are important heteropolysaccharides in the extracellular matrix. These heteropolysaccharides usually are formed by the repetition of a disaccharide unit of an aminosugar and an acid sugar.
A typical example

Other common constituents are sulfate groups linked to certain monosaccharides. Usually heteropolysaccharides are associated with proteins forming proteoglycans, glycosaminoglycans or mucopolysaccharides (since they are abundant in mucous secretions). As a group, they perform diverse functions: structural, water metabolism regulation (as a reservoir of water), cellular cement, biological sieve, biological lubricant, docking sites for growth factors, among other functions.
Established specific functions of some glycosaminoglycans are:
Hyaluronic Acid (Hyaluronate): It is a lubricant in the synovial fluid of joints,
give consistency to vitreous humor, contributes to tensile strength and elasticity of cartilages and tendons (Answer to C-O6)
Chondroitin Sulfates: contributes to tensile strength and elasticity of cartilages, tendons, ligaments and walls of aorta.
Dermatan sulfate (former chondroitin sulfate B) is found mainly in skin, but also is in vessels, heart, lungs. It may be related to coagulation and vascular diseases and other conditions.
Keratan sulfate: Present in cornea, cartilage bone and a variety of other structures as nails and hair.
It is a potent natural anticoagulant produced in the Mast Cells that causes antithrombin bind to thrombin and produce inhibition of blood coagulation.
Glycosaminoglycans are synthesized in the ER and Golgi. They are degraded by lysosomal hydrolases. A deficiency of one of the hydrolases results in a mucopolysaccharidosis. These are hereditary disorders in which glycosaminoglycans accumulate in tissues, causing symptoms such as skeletal and extracellular matrix deformities, and mental retardation.
Examples of these genetic diseases are Hunter and Hurler syndromes.
These diseases, caused by different enzyme deficits, are characterized by physical deformities, mental retardation and disturbances in the degradation of heparan sulfate and dermatan sulfate.

No comments:
Post a Comment