N X S Y R F Q V W C B Y Q E R
N I L T O O H G O W I K W N Z
D K V L P S L C Y U O D N I L
E Q A A L R S Q W L T K L X V
N T V T L M A U I O I X K O G
O S T D K F N R N J N Y O D W
N A H Q S S O I F L X O H I I
F I D M K A M B R C Y M L R W
V Q A R H A M D I C K W U Y T
Q X V C L T W Y W R O X Q P Q
O A D A I T H I A M I N E T P
A F B Q Z N E Z T S V Q H Z V
P O S S N W A T Y M U U C M U
C B Q X T Z V B K X O G Q S W
P E W M X E J S F J P R H H Q
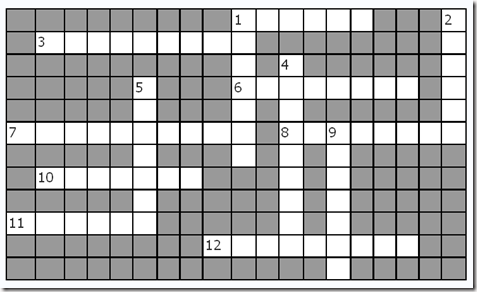
Puzzle 2: Carbohydrate Crossword
(Tip: save the crossword as a picture and print it. Then use a pencil to solve it)

Across
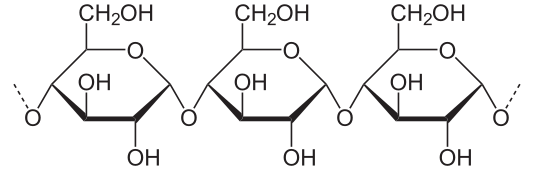
3. Minor disaccharide that can be obtained from starch hydrolysis
5. The main kind of carbohydrate in foods. Also the way in which glucose is stored in plants.
7. The way in which glucose is stored in animals
12. The ketotriose
14. A ketohexose
15. Milk sugar
16. Pentose forming DNA
17. A polyalcohol related to the formation of cataracts in diabetic patients
18. An amino sugar
19. A tetrose
20. A polyalcohol related to the formation of cataracts in patients with galactosemia
21. C-2 epimer of glucose
Down
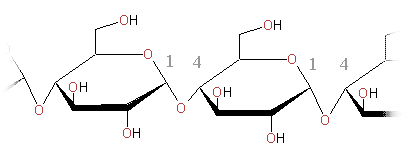
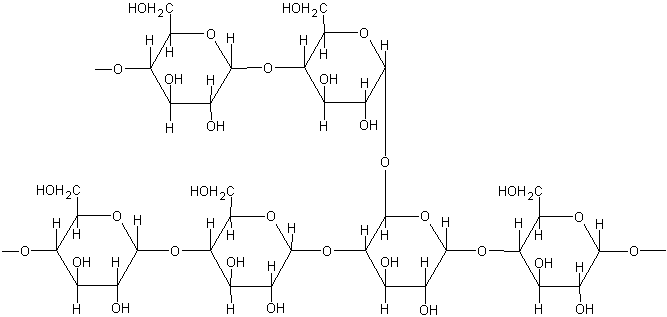
1. The lineal molecule found in starch granules
2. A heteropolysaccharide with anticoagulant effects.
4. A heptose
5. Table sugar
6. An aldohexose found in lactose
8. “Blood sugar”
9. An aldotriose
10. The branched molecule forming starch granules
11. Pentose forming RNA
13. Polysaccharide with a structural rol in plants
21. Main disaccharide obtained from starch hydrolysis
Puzzle 3: Organize the tiles!
When you organize these tiles, you will find a phrase describing a feature of biological catalysts, that in fact is common to all catalysts:

Puzzle4: Solve this amino acid crossword:
 ACROSS
ACROSS1. amino acid positively charged at physiological pH
3. acidic amino acid
6. amino acid that can form disulfide bridges
7. aromatic amino acid
8. basic amino acid
10. polymer of amino acids
11. branched chain amino acid
12. amino acid with OH group in the lateral chain
DOWN
1. apolar amino acid
2. polar, non charged, amino acid
4. negatively charged amino acid at physiological pH
5. imino acid
9. the smallest amino acid
Puzzle 5: Find the names of ten carbohydrates:

Puzzle 6: Find the metabolites of the Krebs Cycle:

SOLUTIONS
Puzzle 1:SOLUTION TO CARBOHYDRATE PUZZLE
GLUCOSE GLYCOGEN MALTOSE RIBOSE
FRUCTOSE ERYTHROSE SUCROSE GLYCERALDEHYDE
GALACTOSE LACTOSE

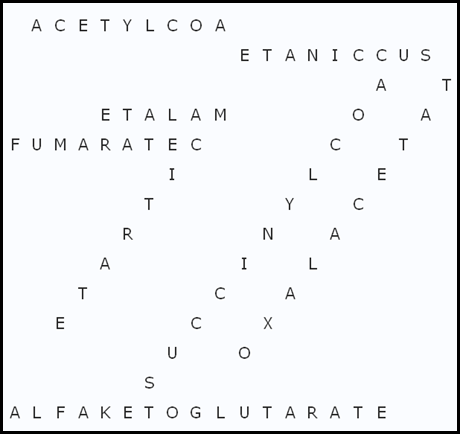
Puzzle2: SOLUTION TO KREBS CYCLE METABOLITES PUZZLE:
SUCCINYLCOA SUCCINATE ALFAKETOGLUTARATE
MALATE CITRATE FUMARATE
ISOCITRATE OXALACETATE ACETYLCOAA
Puzzle 3: Solution to the Tiles problem about biological catalysts
Organizing Tiles: Q: When you organize these tiles, you will find a phrase describing a feature of biological catalysts, that in fact is common to all catalysts
Puzzle 4: ENZ/YME/S D/O N/OT /CHA/NGE/ TH/E E/QUI/LIB/RIU/M O/F T/HE / REA/CTI/ ONS
Puzzle 5: Solution to:Find seven vitaminsBIOTIN
COBALAMIN
FOLATO
NIACIN
PYRIDOXINE
RIBOFLAVIN
THIAMINE
N + + + + F + + + + B + + E +
+ I + + O + + + + + I + + N +
+ + V L + + + + + + O + + I +
+ + A A + + + + + + T + + X +
+ T + + L + + + + + I + + O +
O + + + + F + + N + N + + D +
N + + + + + O I + + + + + I +
+ I + + + + M B + + + + + R +
+ + A + + A + + I + + + + Y +
+ + + C L + + + + R + + + P +
+ + + A I T H I A M I N E + +
+ + B + + N + + + + + + + + +
+ O + + + + + + + + + + + + +
C + + + + + + + + + + + + + +
+ + + + + + + + + + + + + + +
(Over,Down,Direction)
BIOTIN(11,1,S)
COBALAMIN(1,14,NE)
FOLATO(6,1,SW)
NIACIN(1,7,SE)
PYRIDOXINE(14,10,N)
RIBOFLAVIN(10,10,NW)
THIAMINE(6,11,E)